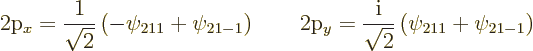|
|
|
|
|
Next: 4.4 Expectation Value and Standard Deviation |
|
This section examines the critically important case of the hydrogen atom. The hydrogen atom consists of a nucleus which is just a single proton, and an electron encircling that nucleus. The nucleus, being much heavier than the electron, can be assumed to be at rest, and only the motion of the electron is of concern.
The energy levels of the electron determine the photons that the atom will absorb or emit, allowing the powerful scientific tool of spectral analysis. The electronic structure is also essential for understanding the properties of the other elements and of chemical bonds.
The first step is to find the Hamiltonian of the electron. The
electron experiences an electrostatic Coulomb attraction to the oppositely charged nucleus. The
corresponding potential energy is
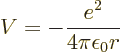 |
(4.28) |
| (4.29) |
| (4.30) |
Unlike for the harmonic oscillator discussed earlier, this potential
energy cannot be split into separate parts for Cartesian coordinates
![]() ,
,![]() ,
,![]() .
.![]()
![]()
![]() -
-![]()
![]() -
-![]() .
.
To get the Hamiltonian, you need to add to this potential energy the kinetic energy operator ![]() .
.
| (4.32) |
It may be noted that the small proton motion can be corrected for by
slightly adjusting the mass of the electron to be an effective 9.104 4 1![]()
Key Points
- To analyze the hydrogen atom, you must use spherical coordinates.
- The Hamiltonian in spherical coordinates has been written down. It is (4.31).
This subsection describes in general lines how the eigenvalue problem for the electron of the hydrogen atom is solved. The basic ideas are like those used to solve the particle in a pipe and the harmonic oscillator, but in this case, they are used in spherical coordinates rather than Cartesian ones. Without getting too much caught up in the mathematical details, do not miss the opportunity of learning where the hydrogen energy eigenfunctions and eigenvalues come from. This is the crown jewel of quantum mechanics; brilliant, almost flawless, critically important; one of the greatest works of physical analysis ever.
The eigenvalue problem for the Hamiltonian, as formulated in the
previous subsection, can be solved by searching for solutions ![]()
![]()
![]()
![]() .
.![]()
![]()
![]() .
.![]() ,
,![]() ,
,![]()
![]()
![]()
![]()
![]()
![]()
![]() ,
,![]()
![]()
![\begin{displaymath}
\begin{array}{r}
\displaystyle
\left[
- \frac{\hbar^2}{2...
...heta\Phi\; \\ [15pt]
\displaystyle= E R\Theta\Phi
\end{array}\end{displaymath}](img659.gif)
![\begin{displaymath}
\frac{1}{\Theta\Phi}
\left[
-\frac{\hbar^2}{\sin\theta}
...
...ial^2}{\partial \phi^2}
\right]
\Theta\Phi
= E_{\theta\phi}
\end{displaymath}](img663.gif)
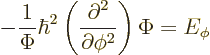
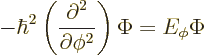
In fact, the solution to this final problem has already been given,
since the operator involved is just the square of the angular momentum
operator ![]()
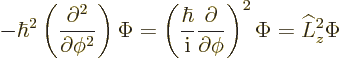
The eigenvalue problem (4.34) for ![]()
![]()
![]()
Returning now to the solution of the original eigenvalue problem
(4.33), replacement of the angular terms by
![]()
![]()
![]()
![]()
It turns out that the solutions of the radial problem can be numbered
using a third quantum number, ![]() ,
,![]() ,
,
| (4.35) |
In terms of these three quantum numbers, the final energy
eigenfunctions of the hydrogen atom are of the general form:
Bohr radiusand has the value
The energy eigenvalues are much simpler and more interesting than the
eigenfunctions; they are
You may wonder why the energy only depends on the principal quantum
number ![]() ,
,![]()
![]() .
.![]() -
-![]()
![]()
![]() .
.
Since the lowest possible value of the principal quantum number ![]()
![]()
![]() .
.
Key Points
- Skipping a lot of math, energy eigenfunctions
and their energy eigenvalues have been found.
- There is one eigenfunction for each set of three integer quantum numbers
,
and ,
satisfying The number .
is called the principal quantum number.
- The typical length scale in the solution is called the Bohr radius
which is about half an Ångstrom. ,
- The derived eigenfunctions
are eigenfunctions of
angular momentum, with eigenvalue ;
- square angular momentum, with eigenvalue
;
- energy, with eigenvalue
.
- The energy values only depend on the principal quantum number
.
- The ground state is
.
Use the tables for the radial wave functions and the spherical harmonics to write down the wave function
Check that the state is normalized. Note: ![]()
![]()
![]() .
.
Use the generic expression
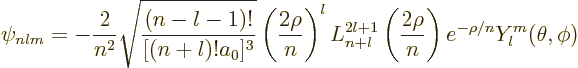
Plug numbers into the generic expression for the energy eigenvalues,
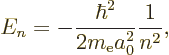
The only energy values that the electron in the hydrogen atom can have
are the “Bohr energies” derived in the previous subsection:
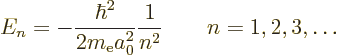
To aid the discussion, the allowed energies are plotted in the form of an energy spectrum in figure 4.8. To the right of the lowest three energy levels the values of the quantum numbers that give rise to those energy levels are listed.
The first thing that the energy spectrum illustrates is that the energy levels are all negative, unlike the ones of the harmonic oscillator, which were all positive. However, that does not mean much; it results from defining the potential energy of the harmonic oscillator to be zero at the nominal position of the particle, while the hydrogen potential is instead defined to be zero at large distance from the nucleus. (It will be shown later, chapter 7.2, that the average potential energy is twice the value of the total energy, and the average kinetic energy is minus the total energy, making the average kinetic energy positive as it should be.)
A more profound difference is that the energy levels of the hydrogen atom have a maximum value, namely zero, while those of the harmonic oscillator went all the way to infinity. It means physically that while the particle can never escape in a harmonic oscillator, in a hydrogen atom, the electron escapes if its total energy is greater than zero. Such a loss of the electron is called “ionization” of the atom.
There is again a ground state of lowest energy; it has total energy
electron voltis 1.6 1
The ionization energy of the hydrogen atom is 13.6 eV; this is the minimum amount of energy that must be added to raise the electron from the ground state to the state of a free electron.
If the electron is excited from the ground state to a higher but still bound energy level, (maybe by passing a spark through hydrogen gas), it will in time again transition back to a lower energy level. Discussion of the reasons and the time evolution of this process will have to wait until chapter 7. For now, it can be pointed out that different transitions are possible, as indicated by the arrows in figure 4.8. They are named by their final energy level to be Lyman, Balmer, or Paschen series transitions.
The energy lost by the electron during a transition is emitted as a quantum of electromagnetic radiation called a photon. The most energetic photons, in the ultraviolet range, are emitted by Lyman transitions. Balmer transitions emit visible light and Paschen ones infrared.
The photons emitted by isolated atoms at rest must have an energy very
precisely equal to the difference in energy eigenvalues; anything else
would violate the requirement of the orthodox interpretation that only
the eigenvalues are observable. And according to the “Planck-Einstein relation,” the photon’s energy equals the
angular frequency ![]()
![]() :
:
(To be sure, the spectral frequencies are not truly mathematically
exact numbers. A slight spectral broadening
is
unavoidable because no atom is truly isolated as assumed here; there
is always some radiation that perturbs it even in the most ideal empty
space. In addition, thermal motion of the atom causes Doppler shifts.
In short, only the energy eigenvalues are observable, but exactly what
those eigenvalues are for a real-life atom can vary slightly.)
Atoms and molecules may also absorb electromagnetic energy of the same frequencies that they can emit. That allows them to enter an excited state. The excited state will eventually emit the absorbed energy again in a different direction, and possibly at different frequencies by using different transitions. In this way, in astronomy atoms can remove specific frequencies from light that passes them on its way to earth, resulting in an absorption spectrum. Or instead atoms may scatter specific frequencies of light in our direction that was originally not headed to earth, producing an emission spectrum. Doppler shifts can provide information about the thermal and average motion of the atoms. Since hydrogen is so prevalent in the universe, its energy levels as derived here are particularly important in astronomy. Chapter 7 will address the mechanisms of emission and absorption in much greater detail.
Key Points
- The energy levels of the electron in a hydrogen atom have a highest value. This energy is by convention taken to be the zero level.
- The ground state has a energy 13.6 eV below this zero level.
- If the electron in the ground state is given an additional amount of energy that exceeds the 13.6 eV, it has enough energy to escape from the nucleus. This is called ionization of the atom.
- If the electron transitions from a bound energy state with a higher principal quantum number
to a lower one it emits radiation with an angular frequency ,
given by
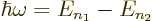
- Similarly, atoms with energy
may absorb electromagnetic energy of such a frequency.
If there are infinitely many energy levels ![]() ,
,![]() ,
,![]() ,
,![]() ,
,![]() ,
,![]() ,
,
What is the value of energy level ![]() ?
?![]() ?
?
Based on the results of the previous question, what is the color of the light emitted in a Balmer transition from energy ![]()
![]() ?
?![]()
![]() ,
,![]()
![]()
![]()
![]()
What is the color of the light emitted in a Balmer transition from an energy level ![]()
![]()
![]() ?
?
The appearance of the energy eigenstates will be of great interest in understanding the heavier elements and chemical bonds. This subsection describes the most important of them.
It may be recalled from subsection 4.3.2 that there is one
eigenfunction ![]()
![]()
![]()
![]()
![]() -
-
For the ground state, with the lowest energy ![]() ,
,![]()
![]()
![]()
![]()
![]() ;
;
The expression for the wave function of the ground state is (from the
results of subsection 4.3.2):
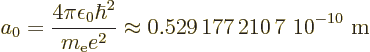 |
(4.41) |
The square magnitude of the energy states will again be displayed as
grey tones, darker regions corresponding to regions where the electron
is more likely to be found. The ground state is shown this way in
figure 4.9; the electron may be found within a blob size
that is about three times the Bohr radius, or roughly an Ångstrom,
(1![]()
It is the quantum mechanical refusal of electrons to restrict
themselves to a single location that gives atoms their size. If
Planck's constant ![]()
The ground state probability distribution is spherically symmetric: the probability of finding the electron at a point depends on the distance from the nucleus, but not on the angular orientation relative to it.
The excited energy levels ![]() ,
,![]() ,
,![]()
Figure 4.10 shows energy eigenfunction ![]() .
.![]() ,
,![]()
The state ![]()
2s
state. The 2 indicates that it is a state with
energy ![]() .
.spherically symmetric.
Similarly,
the ground state ![]()
1s
, having the lowest energy ![]() .
.
States which have azimuthal quantum number ![]()
![]()
![]()
2p
states. As first example of such a state, figure 4.11 shows
![]() .
.![]() -
-![]()
![]() -
-
Since the wave function squeezes close to the ![]() -
-2
state. Think “points
along the ![]()
![]() -
-
Figure 4.12 shows the other two 2p
states,
![]()
![]() .
.![]() -
-
Eigenfunctions ![]() ,
,![]() ,
,![]() ,
,![]()
![]()
![]()
![]() 3
3
In particular, the torus-shaped eigenfunctions ![]()
![]()
![]()
![]() ;
;
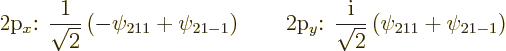 |
(4.42) |
These two states are shown in figure 4.13; they look exactly
like the pointer
state 2![]()
![]() -
-![]() -
-![]() -
-![]() -
-![]()
![]()
Note that unlike the two original states ![]()
![]() ,
,![]()
![]()
![]() -
-![]() -
-![]()
![]()
![]() .
.![]() ,
,![]() ,
,![]()
So, the four independent eigenfunctions at energy level ![]()
![]() ,
,![]() ,
,![]() ,
,
But even that is not always ideal; as discussed in chapter
5.11.4, for many chemical bonds, especially those involving the
important element carbon, still different combination states called
hybrids
show up. They involve combinations of
the 2s and the 2p states and therefore have uncertain
square angular momentum as well.
Key Points
- The typical size of eigenstates is given by the Bohr radius, making the size of the atom of the order of an Å.
- The ground state
or 1s state, is nondegenerate: no other set of quantum numbers ,
produces energy .
- All higher energy levels are degenerate, there is more than one eigenstate producing that energy.
- All states of the form
including the ground state, are spherically symmetric, and are called s states. The ground state ,
is the 1s state, is the 2s state, etcetera.
- States of the form
are called p states. The basic 2p states are ,
and ,
.
- The state
is also called the 2 p state, since it squeezes itself around theaxis. -
- There are similar 2
p and 2p states that squeeze around theand axes. Each is a combination of and .
- The four spatial states at the
energy level can therefore be thought of as one spherically symmetric 2s state and three 2p pointer states along the axes.
- However, since the
energy level is degenerate, eigenstates of still different shapes are likely to show up in applications.
At what distance ![]()
![]()
Check from the conditions
basicsolutions
Check that the states